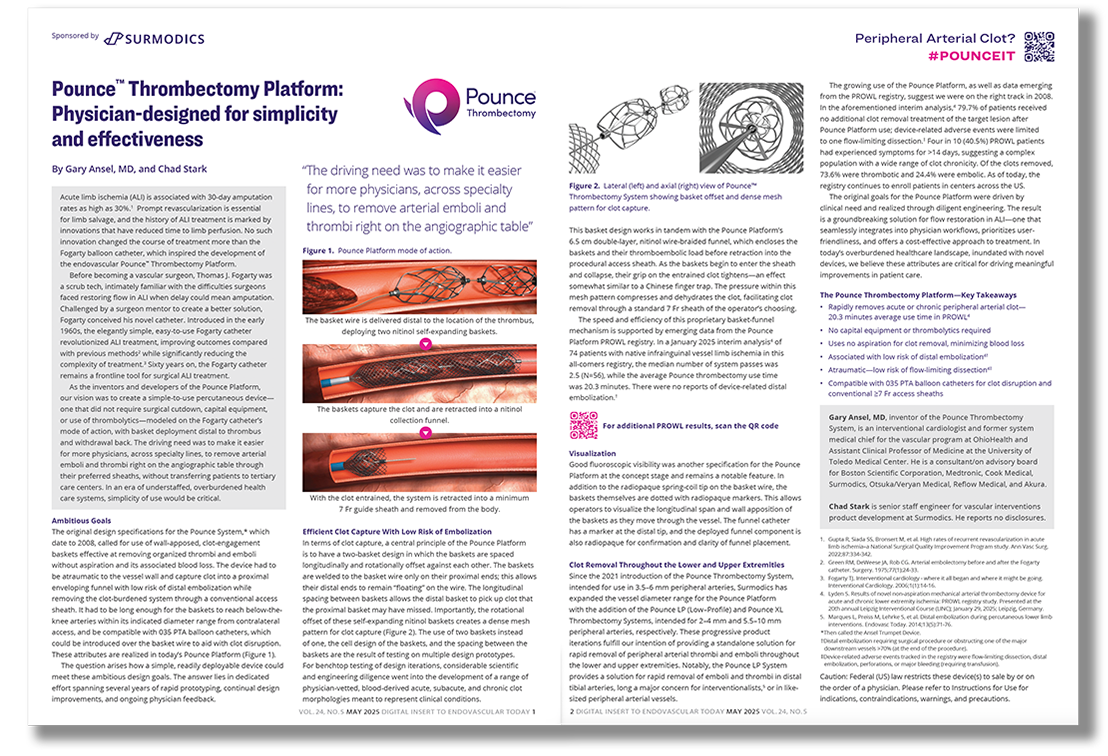
Capture peripheral arterial clot
—on the spot
With a combined vessel diameter range of 2–10mm, the Pounce™ Thrombectomy Platform provides a standalone solution for rapid removal of peripheral arterial thrombi and emboli throughout the lower and upper extremities.
2–10mm

COMBINED VESSEL DIAMETER RANGE
2–4mm
3.5–6mm
5.5–10mm
Rapid removal of acute or chronic thrombi or emboli without thrombolysis
NO aspiration used for clot removal–minimizes blood loss
NO capital equipment
NEW CASE REPORT
5.5–10mm vessel diameter
Removing subacute
CFA thrombus
The Pounce™ XL Thrombectomy System demonstrated versatility by enabling the deployment of a .035” PTA balloon catheter over the basket wire to help loosen the wall-adherent material.
Post-Pounce XL
System angiogram
Pre-procedure
angiogram
ENDOVASCULAR TODAY
READ THE ARTICLE
The Pounce™ Thrombectomy Platform: Physician-designed for simplicity and effectiveness

SAFETY
0% 1.4%
Distal
embolization*
Device-related adverse events†
- Distal embolization requiring surgical procedure or obstructing one of the major downstream vessels >70% (at the end of the procedure).
- Flow-limiting dissection.
The PROWL registry is an open-label, retrospective, multicenter, U.S. study of the Pounce™ Thrombectomy Platform for the non-surgical removal of emboli and thrombi in the peripheral arterial vasculature. NCT05868161
Interim results from 74 patients with native infrainguinal vessel limb ischemia.1
SPEED
20.3min
Average Pounce Platform use time
EFFECTIVENESS
79.7%
No additional clot removal treatment of the target lesion after use
ECONOMICS
67.6%
Avoided ICU admission
Real world data
- Lyden S. Results of novel non-aspiration mechanical arterial thrombectomy device for acute and chronic lower extremity ischemia: PROWL registry study. 20th annual Leipzig Interventional Course (LINC); January 29, 2025; Leipzig, Germany.
Real world data
The PROWL registry is an open-label, retrospective, multicenter, U.S. study of the Pounce™ Thrombectomy Platform for the non-surgical removal of emboli and thrombi in the peripheral arterial vasculature. NCT05868161
Interim results from 74 patients with native infrainguinal vessel limb ischemia.1
SPEED
20.3min
Average Pounce Platform use time
EFFECTIVENESS
79.7%
No additional clot removal treatment
of the target lesion after use
ECONOMICS
67.6%
Avoided ICU admission
SAFETY
0% Distal embolization*
1.4% Device-related adverse events†
- Lyden S. Results of novel non-aspiration mechanical arterial thrombectomy device for acute and chronic lower extremity ischemia: PROWL registry study. 20th annual Leipzig Interventional Course (LINC); January 29, 2025; Leipzig, Germany.
- Distal embolization requiring surgical procedure or obstructing one of the major downstream vessels >70% (at the end of the procedure).
- Flow-limiting dissection.
When minutes matter – remove acute or chronic thrombi or emboli without aspiration, thrombolytics, or capital equipment with the Pounce™ Thrombectomy Platform.
READ THE SUPPLEMENT
Read the new supplement to learn how top endovascular physicians are capturing peripheral arterial clot–on the spot.
Read past Endovascular Today supplements:
Surmodics Announces Successful Early Clinical Use of the Pounce™ LP Thrombectomy System.
Latest press releases
Surmodics Announces Commercial Release of
Pounce™ XL Thrombectomy System, Enabling Rapid
Non-Surgical Clot Removal in Iliac and Femoral Arteries
Surmodics Announces Successful Early Clinical Use of the Pounce™ XL Thrombectomy System, Suitable for Removal of Thrombi and Emboli from Iliac and Femoral Arteries
The Pounce system PROWL registry has completed first patient enrollment.
Read the single-center study
A published single-center study out of South Carolina evaluated the safety and efficacy of the Pounce system to restore pulsatile flow to ischemic lower extremities.
Intended Use:
The Pounce™ Thrombectomy System is intended for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature.
Contraindications:
The device is contraindicated for use in patients who cannot receive recommended intravenous anticoagulant therapy.